Enola is a symptom checker for undiagnosed rare disease patients and their clinicians. It uses real-world evidence to create a prioritised differential diagnosis (DDx) based on the symptoms and findings of the patient. This helps to narrow-down and prioritise further investigation and testing and ensures statistically significant rare diseases are considered. Combined with real-world patient data and evidence, it has the potential to dramatically reduce the diagnostic odyssey faced by patients with an undiagnosed rare disease, currently between 5 and 7 years.
Take a look at Enola but please remember this is only a demonstration of technical feasability (Proof of Concept) and not a medical device. It is not a sympton checker and it does not include common diseases. It should not be used as a substitute for medical advice from a physician.
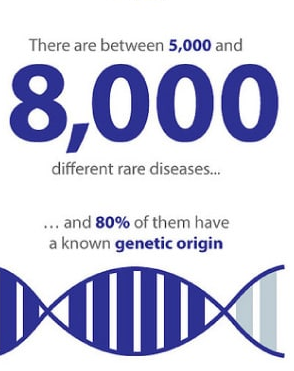
Undiagnosed and rare diseases are a huge challenge for healthcare systems and patients. For more on the scale of the problem, initiatives to improve diagnosis and treatments and training opportunities see:
- Rare X – a collaborative platform for global data sharing and analysis to accelerate treatments for rare disease.
- UDNF aims to foster collaboration among patients, clinicians, and scientists to enhance the quality of life of undiagnosed and ultra-rare disease patients.
- EURODIS Open Academy aims to provide training that empowers not only its member organisations and volunteers, but anyone interested in learning more about the rare disease landscape.
- England Rare Disease action Plan – In this first England Rare Diseases Action Plan, we report on progress made, and take a significant step forward in transforming the collective priorities of the UK Rare Diseases Framework into tangible and concrete action.
- Orphanet maintains the Orphanet nomenclature of rare diseases, essential in improving the visibility of rare diseases in health and research information systems: each disease in Orphanet is attributed a unique and stable identifier, the ORPHAcode.